Russian Periodic Table of Elements - Large Poster (1972)
Medvedeev’s Period Table of Elements
Designed by EV Beketov and H. Shchegelov. Lithograph on paper. Print Code 2-5-2 / 15-72. Measures 120cm x 90cm. Printed in Moscow. Condition: Very good. Some edge damage in places and some creases. Small amount of tape on rear. Stored rolled.
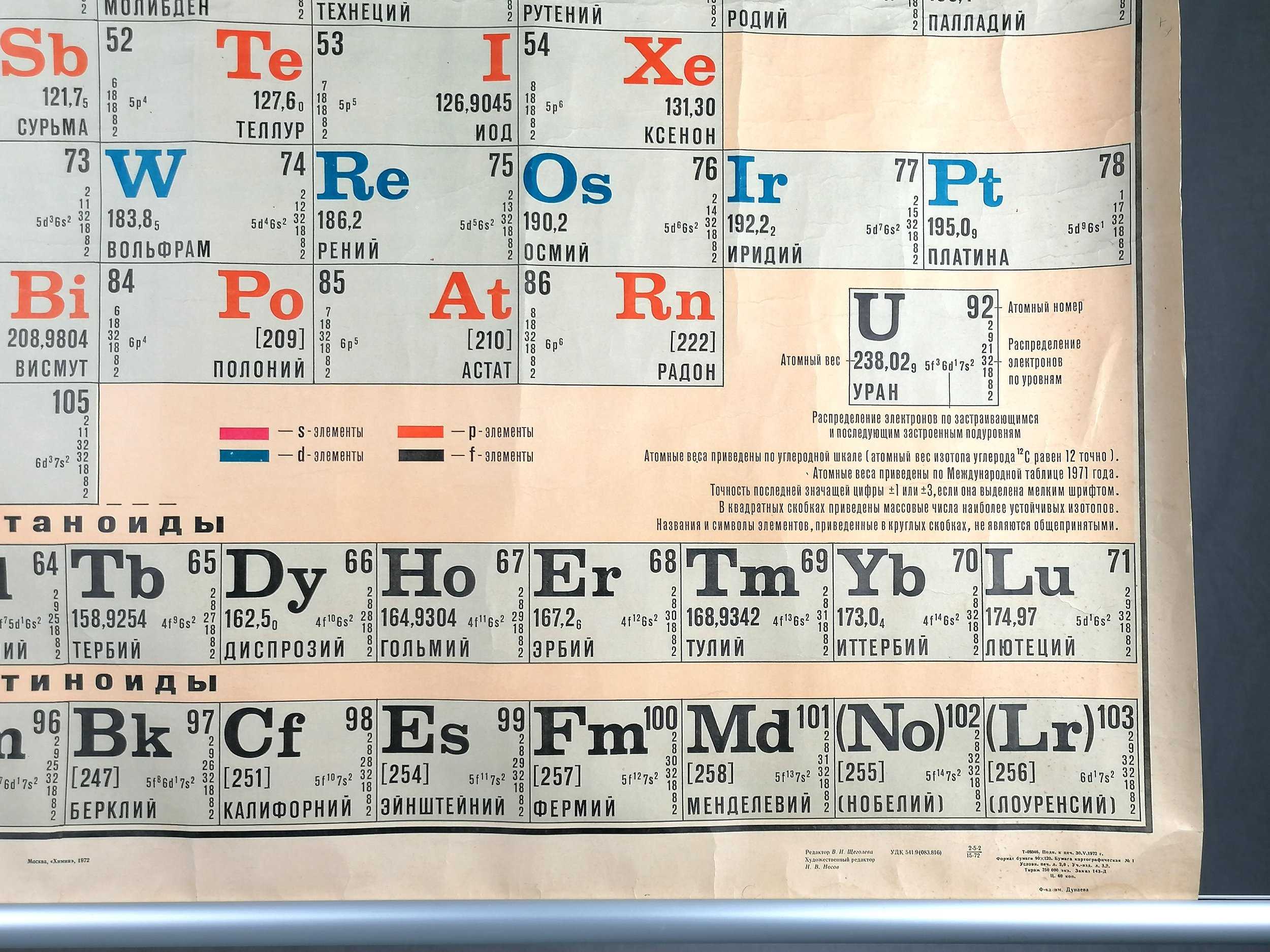
This huge school poster was printed for display in research facilities, laboratories and schools across Russia. Printed in 1972, this detailed periodic table only shows 105 elements - today’s table has 118. It wasn’t until 1974 that the 106th element (Seaborgium) was synthesised. In addition to the atomic number, the atomic weight and electron tiering is also shown for each element.
The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869: he formulated the periodic law as a dependence of chemical properties on atomic mass. Because not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict properties of some of the missing elements.
Controversy arose with elements 102 through 106 in the 1960s and 1970s, as the US and Russia raced to discover them first. Often, both claimed discovery at the same time and in some cases each proposed their own name for the element, creating an element naming controversy that lasted decades. An example of this can be seen in element 104, which in this table is called Kurchatovium (Ku) but was eventually renamed Rutherfordium (Rf) in 1997.
A similar example of this poster can be viewed in this video (at 1:30) https://www.youtube.com/watch?v=1VaY9N7Alq0
A cool piece of academic and chemistry history.
Medvedeev’s Period Table of Elements
Designed by EV Beketov and H. Shchegelov. Lithograph on paper. Print Code 2-5-2 / 15-72. Measures 120cm x 90cm. Printed in Moscow. Condition: Very good. Some edge damage in places and some creases. Small amount of tape on rear. Stored rolled.
This huge school poster was printed for display in research facilities, laboratories and schools across Russia. Printed in 1972, this detailed periodic table only shows 105 elements - today’s table has 118. It wasn’t until 1974 that the 106th element (Seaborgium) was synthesised. In addition to the atomic number, the atomic weight and electron tiering is also shown for each element.
The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869: he formulated the periodic law as a dependence of chemical properties on atomic mass. Because not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict properties of some of the missing elements.
Controversy arose with elements 102 through 106 in the 1960s and 1970s, as the US and Russia raced to discover them first. Often, both claimed discovery at the same time and in some cases each proposed their own name for the element, creating an element naming controversy that lasted decades. An example of this can be seen in element 104, which in this table is called Kurchatovium (Ku) but was eventually renamed Rutherfordium (Rf) in 1997.
A similar example of this poster can be viewed in this video (at 1:30) https://www.youtube.com/watch?v=1VaY9N7Alq0
A cool piece of academic and chemistry history.
Medvedeev’s Period Table of Elements
Designed by EV Beketov and H. Shchegelov. Lithograph on paper. Print Code 2-5-2 / 15-72. Measures 120cm x 90cm. Printed in Moscow. Condition: Very good. Some edge damage in places and some creases. Small amount of tape on rear. Stored rolled.
This huge school poster was printed for display in research facilities, laboratories and schools across Russia. Printed in 1972, this detailed periodic table only shows 105 elements - today’s table has 118. It wasn’t until 1974 that the 106th element (Seaborgium) was synthesised. In addition to the atomic number, the atomic weight and electron tiering is also shown for each element.
The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869: he formulated the periodic law as a dependence of chemical properties on atomic mass. Because not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict properties of some of the missing elements.
Controversy arose with elements 102 through 106 in the 1960s and 1970s, as the US and Russia raced to discover them first. Often, both claimed discovery at the same time and in some cases each proposed their own name for the element, creating an element naming controversy that lasted decades. An example of this can be seen in element 104, which in this table is called Kurchatovium (Ku) but was eventually renamed Rutherfordium (Rf) in 1997.
A similar example of this poster can be viewed in this video (at 1:30) https://www.youtube.com/watch?v=1VaY9N7Alq0
A cool piece of academic and chemistry history.